During the month of October, the world turns pink in dedication of Breast Cancer Awareness Month. Each year, an estimated 1 million cases of breast cancer are diagnosed. Of these, about 10-20% is the aggressive form triple negative breast cancer (TNBC). Unfortunately, the standard chemotherapies have shown to be ineffective on TNBC thus we were curious how the drug development pipeline looked like for this particular subgroup of breast cancer, TNBC.
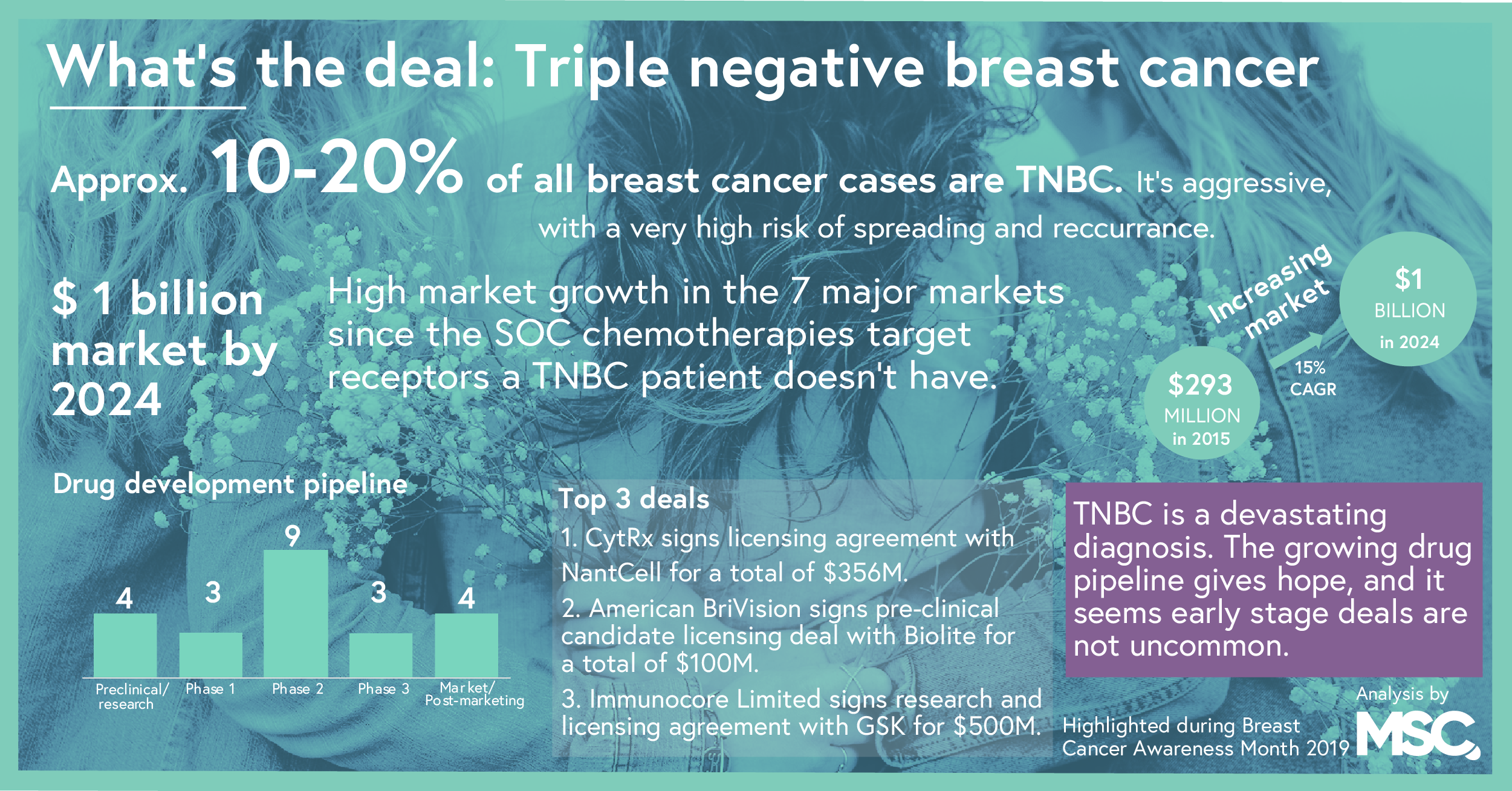
The market for TNBC is projected to reach 1 billion USD by 2024 across the US, Japan and five major EU markets. Currently, the only treatment option is the standard chemotherapy, why there is now a big interest in finding new treatments. Primary drivers of this rapid expansion include the introduction and uptake of next-generation targeted treatments and immunotherapies. Poly (ADP-ribose) polymerase inhibitors and programmed death-1 (PD-1)/PD ligand-1 inhibitors are expected to be the best-selling classes of drugs over the next few years.
What is triple negative breast cancer?
TNBC is an aggressive type of breast cancer, where not only the risk of the cancer spreading is very high but also the recurrence rate. Breast cancer tumors where the cells are negative for the three major receptors most known as the driving factors behind a majority of breast cancer; estrogen, progesterone and HER2 receptors are classified as triple negative. Notably, studies have shown it to more likely to affect younger people, Hispanics, African Americans, and/or those with a mutation in the BRCA1 gene.
Gap in the treatment landscape
Many of the common therapies for treating breast cancer, such as Tamoxifen or aromatase inhibitors in hormone therapy and drugs that target estrogen, progesterone and HER2, such as Herceptin and Tykerb, are quite ineffective, as triple negative tumors lack the receptors necessary for responding to the treatment. With chemotherapy being one of the few effective treatment options, there is a big interest in finding new drugs for this aggressive disease.
References: National Breast Cancer Foundation. Triple negative breast cancer. Available at: https://www.nationalbreastcancer.org/triple-negative-breast-cancer [Accessed 1 Oct. 2019]; Johns Hopkins Medicine. Triple negative breast cancer. Available at: https://www.hopkinsmedicine.org/breast_center/breast_cancers_other_conditions/triple_negative_breast_cancer.html [Accessed 1 Oct. 2019]; Ismail-Khan R. and Bui M. M. (2010). A Review of Triple-negative Breast Cancer. Cancer Control. 2010;17(3):173-176.; CytRx Corporation (2017). CytRx Corporation Announces Global Strategic License With NantCell Inc. For Aldoxorubicin, An Albumin Mediated Chemotherapeutic. [online] Available at: https://www.prnewswire.com/news-releases/cytrx-corporation-announces-global-strategic-license-with-nantcell-inc-for-aldoxorubicin-an-albumin-mediated-chemotherapeutic-300495897.html [Accessed 4 Oct. 2019].; US Securities and Exchange Commission. Amendment no.1 to form S-1. Available at: https://www.sec.gov/Archives/edgar/data/1173313/000161577416008325/s104643_s1a.htm [Accessed 4 Oct. 2019]; Informa. (2017) Breast cancer: triple-negative. Forecast. Februari 2017. [Online] [Accessed on 1 Oct. 2019] https://service.datamonitorhealthcare.com; Informa. (2018) Breast cancer: triple-negative. Epidemiology. May 2018. [Online] [Accessed on 1st October 2019] https://service.datamonitorhealthcare.com; Analysis by MSC based on data from Medtrack and Datamonitor.



